HCl: highly dangerous gas. Corrosive, irritant. IDLH: 50 ppm. Severe effects: >5 ppm. Respiratory damage: pulmonary edema, ARDS. Skin/eye burns. Reacts with metals: H2 gas (explosive). Use PPE, ventilation, gas detectors. LC50 (rat): 3,124 ppm/1h. LD50 (dermal, rabbit): >5,010 mg/kg.
How Does Hydrogen Chloride Affect the Respiratory System?
Exposure to hydrogen chloride can cause severe damage to the respiratory system, even at relatively low concentrations. The gas reacts with the moisture in the mucous membranes, forming hydrochloric acid (HCl(aq)):
HCl(g) + H2O(l) → HCl(aq)
The resulting acid causes irritation and inflammation of the respiratory tract, leading to symptoms such as:
- Coughing and choking sensation
- Shortness of breath and chest pain
- Laryngeal spasms and edema
- Pulmonary edema and acute respiratory distress syndrome (ARDS) in severe cases
The severity of the damage depends on factors such as the concentration of HCl, duration of exposure, and individual susceptibility. The NIOSH Immediately Dangerous to Life or Health (IDLH) concentration for HCl is 50 ppm, while the OSHA Permissible Exposure Limit (PEL) is 5 ppm (ceiling).
What Are the Corrosive Effects of Hydrogen Chloride on Skin and Eyes?
Hydrogen chloride is highly corrosive to skin and eyes due to its ability to form hydrochloric acid upon contact with moisture. The acid rapidly penetrates the skin, causing:
- Redness, irritation, and burning sensation
- Chemical burns and ulceration
- Permanent scarring and tissue damage in severe cases
Eye exposure to HCl can lead to:
- Severe irritation and pain
- Corneal ulceration and opacity
- Permanent vision loss
The extent of the damage depends on the concentration of the gas and the duration of exposure. Immediate decontamination with copious amounts of water is crucial to minimize the corrosive effects.
How Does Hydrogen Chloride React with Metals?
Hydrogen chloride readily reacts with many metals, forming metal chlorides and releasing hydrogen gas:
2HCl(g) + M(s) → MCl2(s) + H2(g)
This reaction can be dangerous for several reasons:
- Hydrogen gas is highly flammable and explosive, with a Lower Explosive Limit (LEL) of 4% and an Upper Explosive Limit (UEL) of 75%.
- Some metal chlorides, such as aluminum chloride (AlCl3) and titanium chloride (TiCl4), are highly reactive and can generate heat and toxic fumes upon contact with water or air.
- The corrosion of metals by HCl can weaken the structural integrity of equipment and containers, leading to leaks and potential exposures.
To minimize the risks associated with HCl-metal reactions, it is essential to:
- Use corrosion-resistant materials, such as glass, Teflon, or Hastelloy C, for handling and storing HCl
- Avoid contact between HCl and reactive metals, such as aluminum, magnesium, and zinc
- Ensure proper ventilation to prevent the accumulation of hydrogen gas
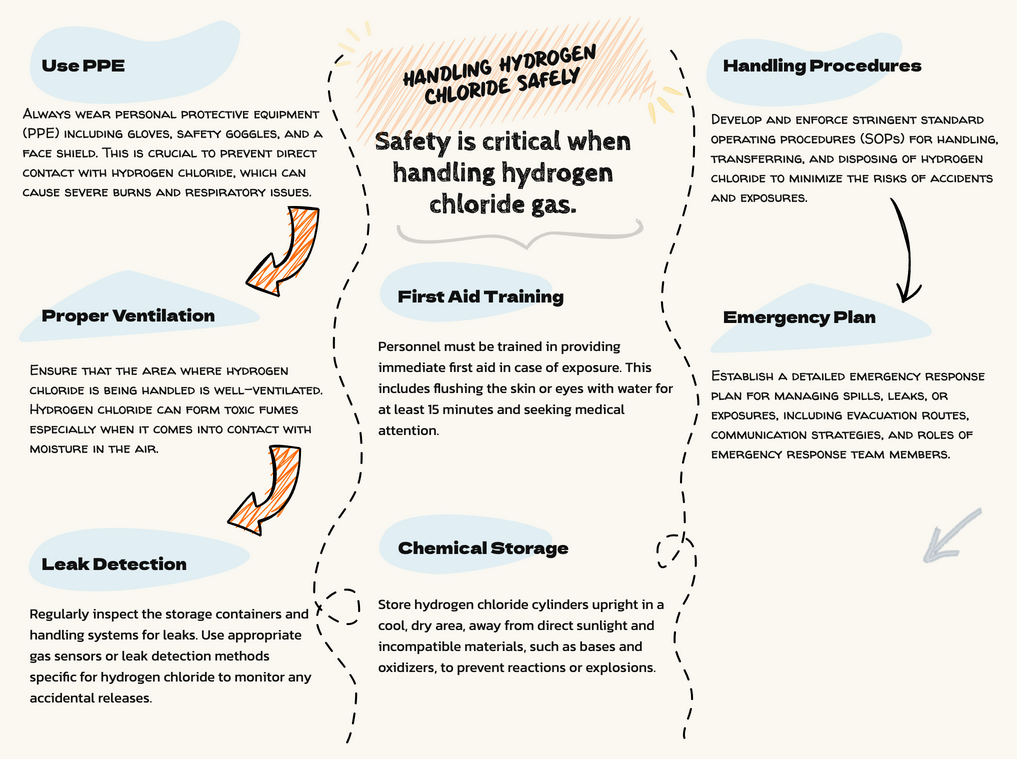
What Safety Precautions Should Be Taken When Handling Hydrogen Chloride?
Due to the hazardous nature of hydrogen chloride, strict safety precautions must be followed when handling the gas:
Use appropriate personal protective equipment (PPE):
- Chemical-resistant gloves (e.g., butyl rubber, Viton)
- Protective eyewear (goggles or face shield)
- Respiratory protection (self-contained breathing apparatus or air-purifying respirator with acid gas cartridges)
- Acid-resistant clothing (apron, boots)
Work in a well-ventilated area or use a fume hood to minimize exposure
- Ensure a minimum face velocity of 100 fpm for fume hoods
- Regularly check the effectiveness of the ventilation system using smoke tubes or anemometers
Implement proper engineering controls
- Use gas detection systems with alarms set at the OSHA PEL (5 ppm)
- Install emergency eyewash stations and safety showers in areas where HCl is handled
Develop and follow standard operating procedures (SOPs) for:
- Safe handling, storage, and disposal of HCl
- Emergency response in case of accidental release or exposure
- Regular maintenance and inspection of safety equipment
Provide regular training to personnel on the hazards of HCl and proper safety procedures
| Concentration (ppm) | Health Effects | Exposure Limits |
|---|---|---|
| 1-5 | Mild irritation | OSHA PEL: 5 ppm (ceiling) |
| 5-10 | Severe irritation, coughing, choking | NIOSH REL: 5 ppm (ceiling) |
| 10-50 | Pulmonary edema, ARDS, skin burns | NIOSH IDLH: 50 ppm |
| >50 | Life-threatening effects, permanent damage | – |